Human Factors
Human factors engineering and usability capabilities to support all project phases
Designing Safe, Effective and User-Friendly Products
Because we have to…
Most medical devices are required by the FDA and international regulatory bodies to have Human Factors Engineering (HFE) deliverables included in the submission to ensure safe and effective use with intended users in their use environments.
Because we want to…
Including HFE in the design process early and often creates better, more usable products that have increased sales, longer lifecycles and decreased service costs.
Because we strive to…
Applying a robust HFE process helps us create the best products, make a difference in overall patient care and user experience, and empower our clients to understand their users and sell more products.
Benefits of Incorporating Human Factors

Compliance: HFE work is required for CE mark and FDA 510(k) new device submissions.

Litigation deterrence: Regulatory bodies hold manufacturers responsible for adverse events due to use error.

Faster time to market: HFE work helps prevent late-stage design changes due to missed user needs and unmitigated use errors. The design is optimized from inception for the intended users, uses, and use environments.

Simplified learning tools: When products are designed for the intended users’ capabilities, learning tools (e.g., training, IFUs) are simpler and reduced in scope.

Increased sales: Greater ease of use boosts sales and enables better competition in the marketplace. Best-to-market trumps first-to-market.

Longer design life: When products are optimized for use, they remain competitive for longer periods of time, avoiding major redesigns.

Lower support costs: Demand for service or support drops when products are easier to learn, use and troubleshoot.
Agile Approaches to Suit Unique Programs
Solving critical usability problems and mitigating use-related risks through creative, efficient research and testing methods
Our Human Factors team utilizes a variety of techniques to meet your program budget and timeline without sacrificing user advocation in product design. With human factors and user experience engineering, a little goes a long way, so we can meet our clients where they need us — from full program support alongside our design and development teams to a standalone validation test, formative evaluation or heuristic analysis.

Integrating Human Factors with Design Capabilities
Prototyping, testing and iterating per user feedback in real-time
At Tensentric, human factors are integrated with our cross-disciplinary design teams. This means that our industrial design group supports our HF studies with fit-for-purpose prototypes with input from our experienced design and development team so our clients can converge on the optimal balance between usability, functional, and business constraints more rapidly.

WORK
WITH US
Interested in how we can help you?
Let’s chat.
Our Thinking
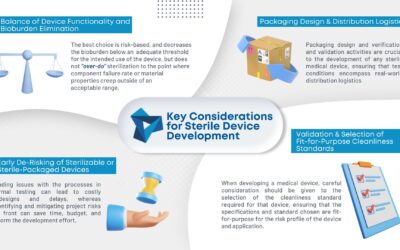
KEY CONSIDERATIONS FOR STERILE DEVICE DEVELOPMENT
As medical device consumables become more complex, so does manufacturing them. Sterilization is becoming an increasing concern in the industry. To ensure the proper sterilization is in place, there...
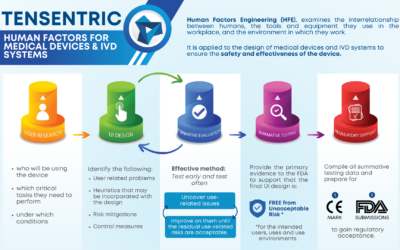
WHAT IS HUMAN FACTORS ENGINEERING AND WHY IS IT IMPORTANT?
Medical devices and IVD systems are developed with the purpose of assisting health care providers diagnose and treat patients to overcome sickness or disease to, in the end, improve their quality of...
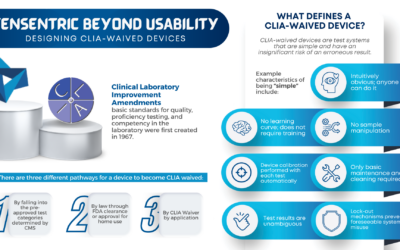
BEYOND USABILITY: DESIGNING CLIA WAIVED DEVICES
Are you ready for the Clinical Laboratory Improvement Amendments waiver? With the large market for laboratory devices growing fast, the benefits of designing for the waiver are even more important...
Team
Our Team Offerings

USER RESEARCH
Risk Analysis

UX DESIGN

FORMATIVE EVALUATIONS & HF VALIDATION (SUMMATIVE) TESTING

REGULATORY SUPPORT
Our Clients
We serve a broad spectrum of clients from early-stage start-ups through large and well-established medical and life sciences OEMs.
Related Insights
WHAT IS HUMAN FACTORS ENGINEERING AND WHY IS IT IMPORTANT?
Medical devices and IVD systems are developed with the purpose of assisting health care providers diagnose and treat patients to overcome sickness or disease to, in the end, improve their quality of...
BEYOND USABILITY: DESIGNING CLIA WAIVED DEVICES
Are you ready for the Clinical Laboratory Improvement Amendments waiver? With the large market for laboratory devices growing fast, the benefits of designing for the waiver are even more important...
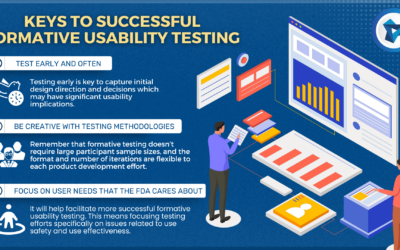
Formative Usability Testing in Medical Device Development
Imagine a medical device development team has done preliminary analyses on who will be using the device.