SERVICES
Turn-key System Design from Initial Concept through Volume Manufacturing
Human Factors
Our best in class Human Factors team helps you optimize the usability and use-safety of your products and maintain compliance with latest US and OUS standards and guidance.
Design & Development
From early concept development and ideation, through detailed design and prototyping, developing custom engineered instruments and consumables is Tensentric’s core competency.
Manufacturing
Tensentric can carry our designs into manufacturing of finished products. In our dedicated manufacturing facility, we focus on high level assembly of highly complex systems during NPI and ramp-to-volume.
INDUSTRIES
Focused on Clients Working to Improve Clinical Outcomes
Expertise
Team
Thinking
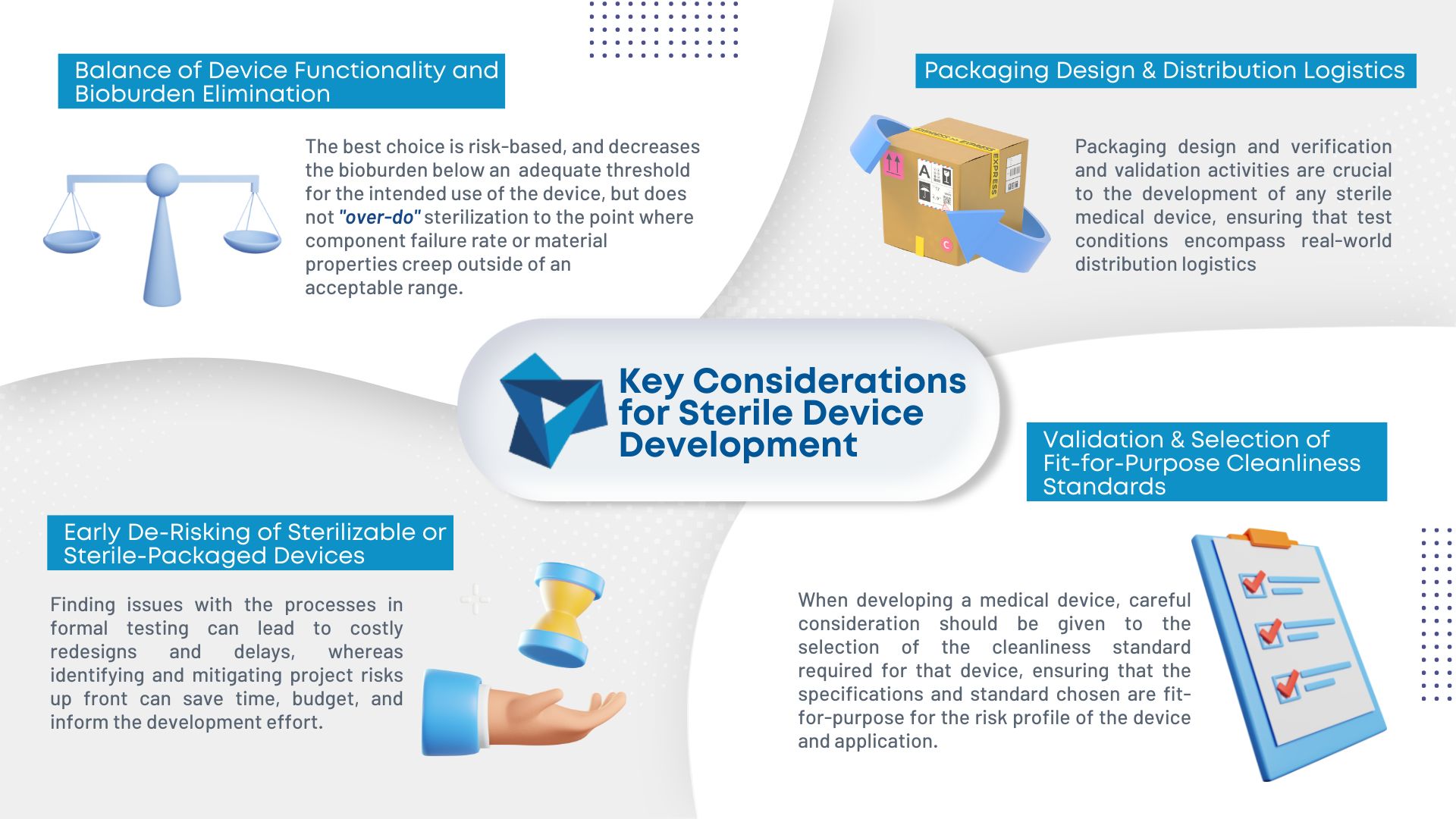
As medical device consumables become more complex, so does manufacturing them. Sterilization is becoming an increasing concern in the industry. To ensure the proper sterilization is in place, there are four key considerations during development: Balance of device functionality and bioburden elimination: Different sterilization methods have different degradation effects on materials making it important to […]
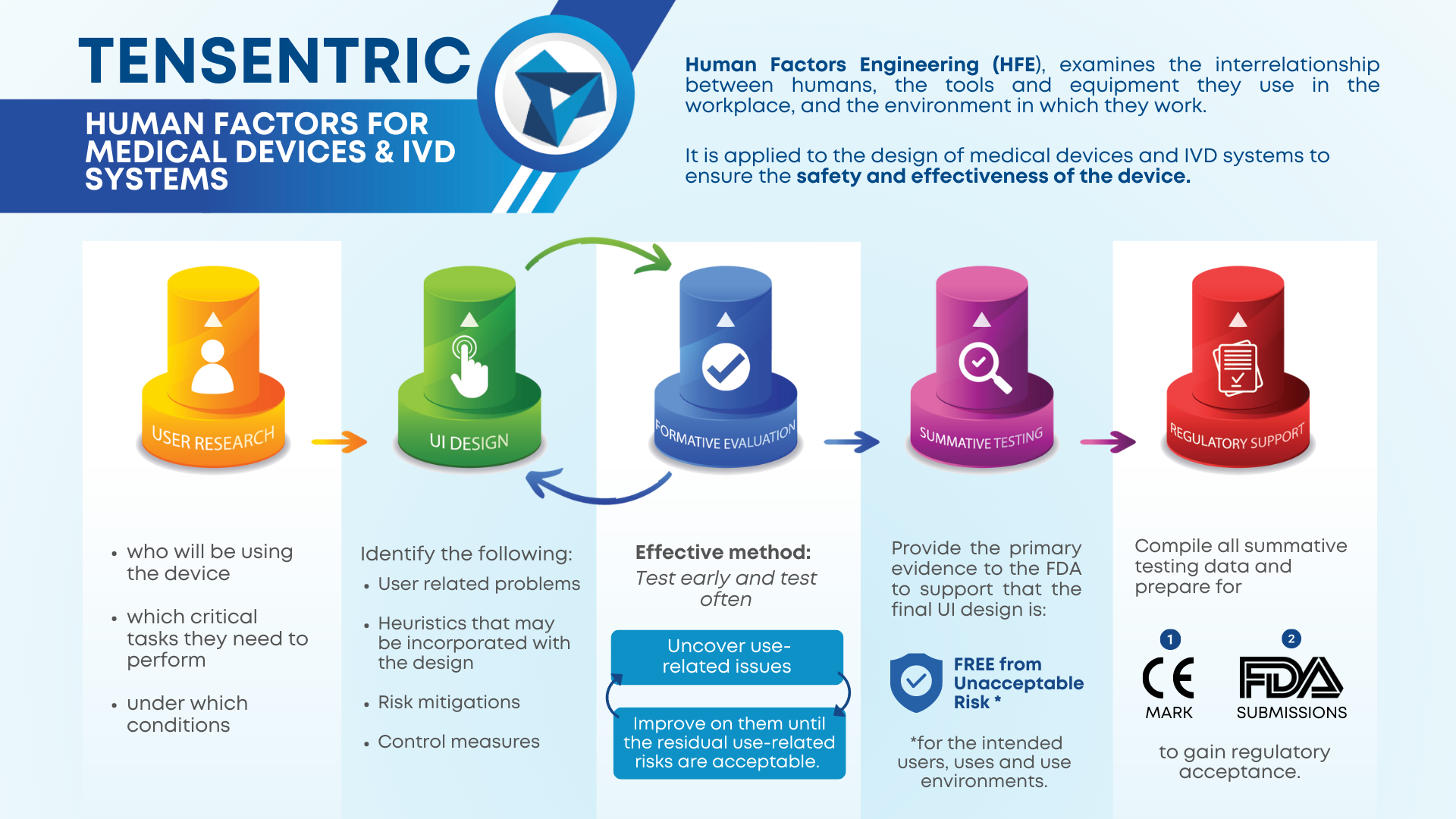
Medical devices and IVD systems are developed with the purpose of assisting health care providers diagnose and treat patients to overcome sickness or disease to, in the end, improve their quality of life. Therefore, it is essential that medical devices and IVD systems be designed as safe and effective for the intended users, uses and […]
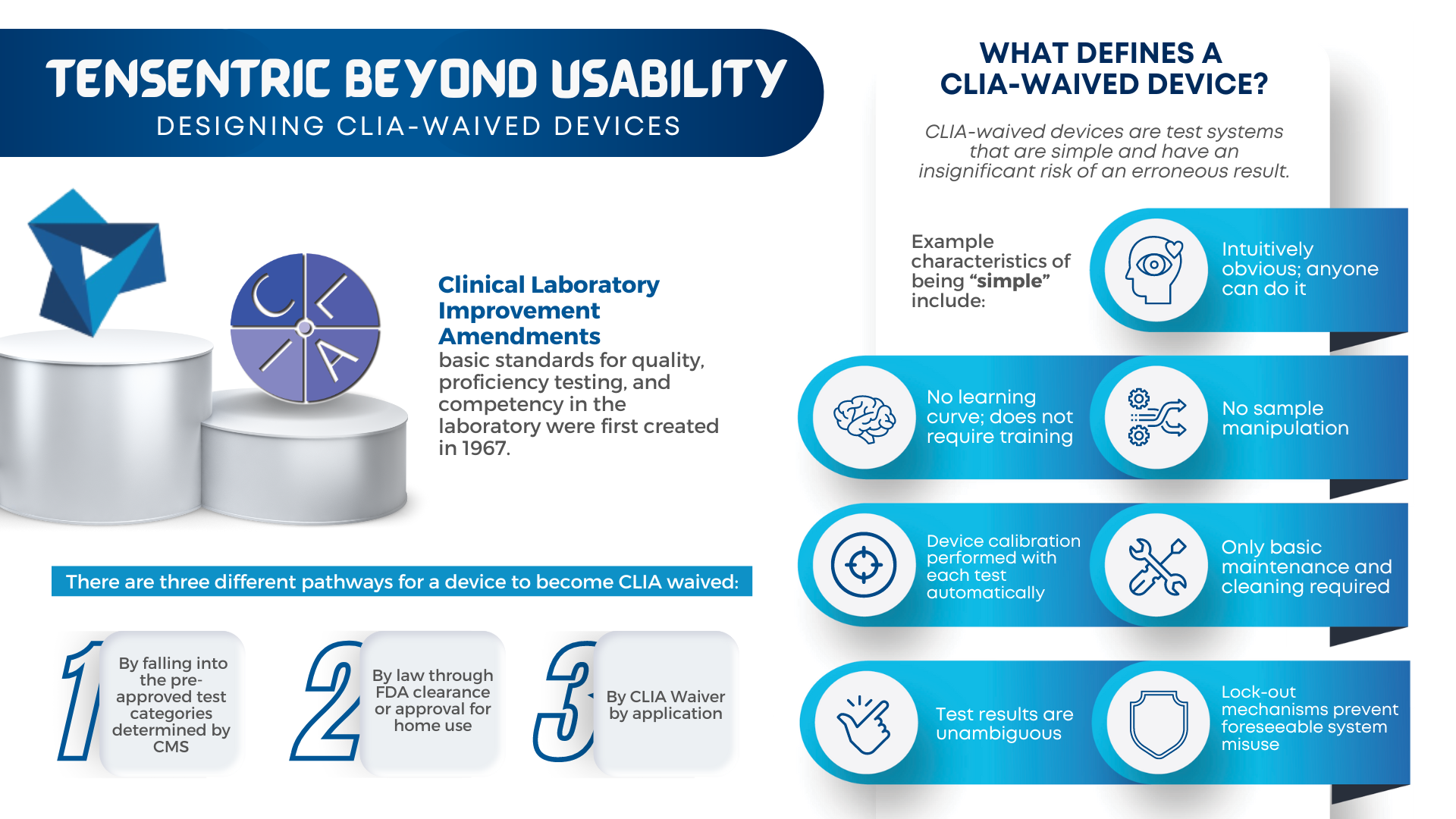
Are you ready for the Clinical Laboratory Improvement Amendments waiver? With the large market for laboratory devices growing fast, the benefits of designing for the waiver are even more important for better and faster patient care. For manufacturers, it vastly broadens the potential market to include non-lab and non-hospital clinics, urgent care centers, medical facilities […]
Our Clients Include…
Contact Us
PROJECT INQUIRIES:
Jeffrey Gentry, Chief Commercial Officer
jeff.gentry@tensentric.com
+1.720.204.0071
MAIN OFFICE:
2900 Center Green Court
Boulder, Colorado 80301
corporate@tensentric.com
+1.303.402.0477
TENSENTRIC MANUFACTURING:
474 South Taylor Ave.
Louisville, CO 80027
mfg@tensentric.com
HUMAN FACTORS & USABILITY:
usability@tensentric.com
CAREER INQUIRIES:
careers@tensentric.com
INTERNSHIP INQUIRIES:
HR & EMPLOYMENT VERIFICATION:
administrative@tensentric.com
+1.303.402.0477
Tensentric is ISO 13485 certified.
This link leads to the machine readable files that are made available in response to the federal Transparency in Coverage Rule and includes negotiated service rates and out-of-network allowed amounts between health plans and healthcare providers. The machine-readable files are formatted to allow researchers, regulators, and application developers to more easily access and analyze data.