Implementing a Fit-for-Purpose Approach
Product development from early concept through manufacturing
Tensentric uses a modified Agile development process we call AgileRx™, a hybrid between a traditional Waterfall Method (which is tiered) and a more flexible, agile approach.
Product realization and development is divided into major milestones, each with its own set of defined deliverables and exit criteria. To complete a milestone, the project team works in short intervals called sprints (usually two weeks long) where tasks are defined and executed.
Operating on a “sprint” cadence improves our ability to provide detailed estimates, track project progress, and identify schedule risks while using a phased approach allows us to apply a consistent rigorous product development process, including risk management, that are key to successful medical device design.
An extension of this fit-for-purpose approach is Tensentric’s Phase Zero, which is a technical and project risk reduction activity prior to formal design controls, where user needs are gathered, concepts are rapidly generated and assessed, functional breadboards and elements are prototyped to demonstrate technical feasibility, and low fidelity industrial design concepts are leveraged to capture human factors feedback early on in development.

Preparing Your Product for Regulated Markets
Safety for clinicians, caregivers and patients
We not only design and create these systems, but we also make sure they align with the FDA and other regulatory bodies’ rigorous standards. It’s all about making sure everything is safe and effective for providers and patients.
We work with our clients to develop a custom design and development plan to pragmatically meet regulatory constraints, from lab devices to cell therapy bioprocessing systems to diagnostic systems and medical devices, in our fully compliant ISO-13485 quality management system.

WORK
WITH US
Interested in how we can help you?
Let’s chat.
Our Thinking
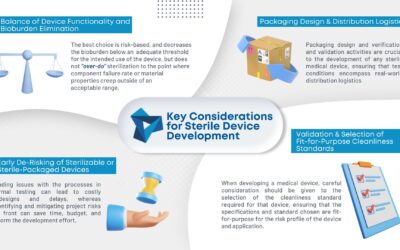
KEY CONSIDERATIONS FOR STERILE DEVICE DEVELOPMENT
As medical device consumables become more complex, so does manufacturing them. Sterilization is becoming an increasing concern in the industry. To ensure the proper sterilization is in place, there...
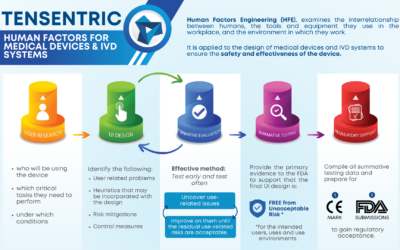
WHAT IS HUMAN FACTORS ENGINEERING AND WHY IS IT IMPORTANT?
Medical devices and IVD systems are developed with the purpose of assisting health care providers diagnose and treat patients to overcome sickness or disease to, in the end, improve their quality of...
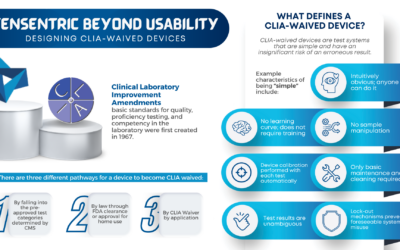
BEYOND USABILITY: DESIGNING CLIA WAIVED DEVICES
Are you ready for the Clinical Laboratory Improvement Amendments waiver? With the large market for laboratory devices growing fast, the benefits of designing for the waiver are even more important...
Our Team Offerings
Human Factors
Concept Ideation & Industrial Design
Mechanical & Electrical Engineering Design
Software & Firmware Development
Design for Reliability & Manufacturability

Assembly and Text Fixture Design & Validation

NPI & Volume Manufacturing
Our Clients
We serve a broad spectrum of clients from early-stage start-ups through large and well-established medical and life sciences OEMs.
Related Insights
KEY CONSIDERATIONS FOR STERILE DEVICE DEVELOPMENT
As medical device consumables become more complex, so does manufacturing them. Sterilization is becoming an increasing concern in the industry. To ensure the proper sterilization is in place, there...
WHAT IS HUMAN FACTORS ENGINEERING AND WHY IS IT IMPORTANT?
Medical devices and IVD systems are developed with the purpose of assisting health care providers diagnose and treat patients to overcome sickness or disease to, in the end, improve their quality of...
BEYOND USABILITY: DESIGNING CLIA WAIVED DEVICES
Are you ready for the Clinical Laboratory Improvement Amendments waiver? With the large market for laboratory devices growing fast, the benefits of designing for the waiver are even more important...