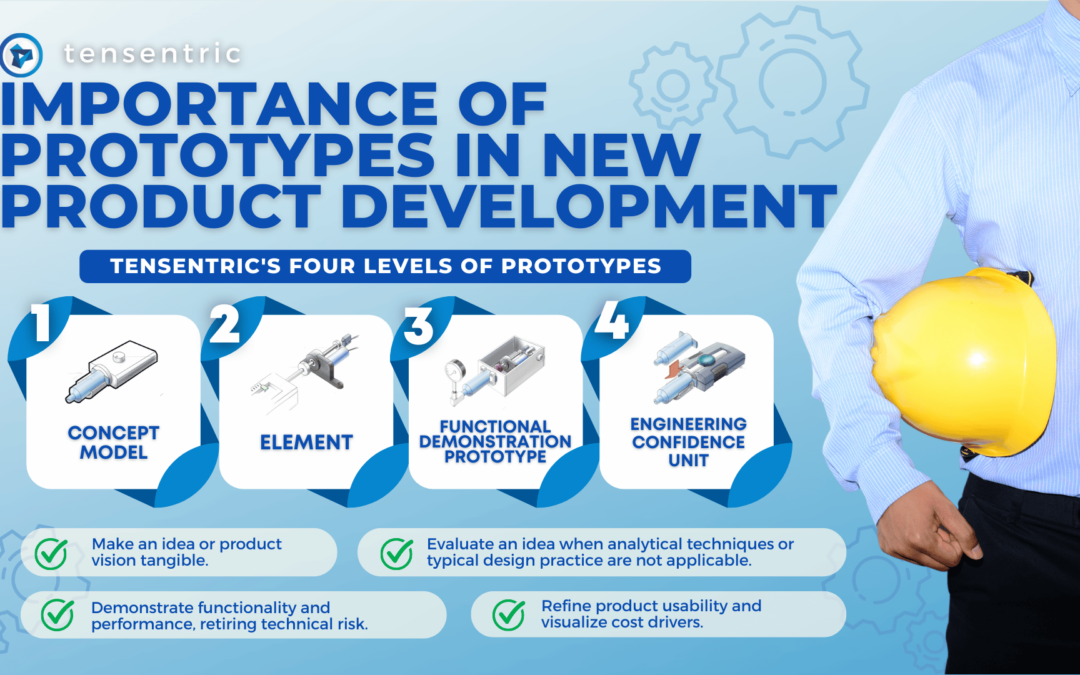
Medical devices and IVD systems are developed with the purpose of assisting health care providers diagnose and treat patients to overcome sickness or disease to, in the end, improve their quality of life. Therefore, it is essential that medical devices and IVD systems be designed as safe and effective for the intended users, uses and use environments. Human Factors Engineering (HFE), which examines the interrelationship between humans, the tools and equipment they use in the workplace, and the environment in which they work, is applied to the design of medical devices and IVD systems to ensure the safety and effectiveness of the device. In fact, in recognition of HFE’s value in ensuring marketed devices are designed to be safe and effective, FDA and other regulatory bodies require HFE during device design and development in order to minimize potential use errors and resulting harm.
The FDA and international bodies expect you to establish a process to achieve reasonable usability to minimize use errors and use associated risks.
Fundamentally, the FDA and other regulatory bodies want to know that users can use the medical device as needed to accomplish their critical tasks. In particular, they are concerned with end users being able to safely and effectively use the device as needed:
- In the intended use environment(s)
- With the designed user interface (UI) and instructions for use (IFU)
- Without committing errors that are a result of the UI design
- Without the outcome resulting in or leading to harm to the patient
In the years since the FDA’s HFE guidance was released in 2000, FDA been increasingly scrutinizing new device submissions for HFE and even rejecting new device submissions based on HFE! Therefore, it is essential that you apply a thorough HFE process with all medical device designs.
HOW DO I EVEN BEGIN THE HUMAN FACTORS PROCESS?
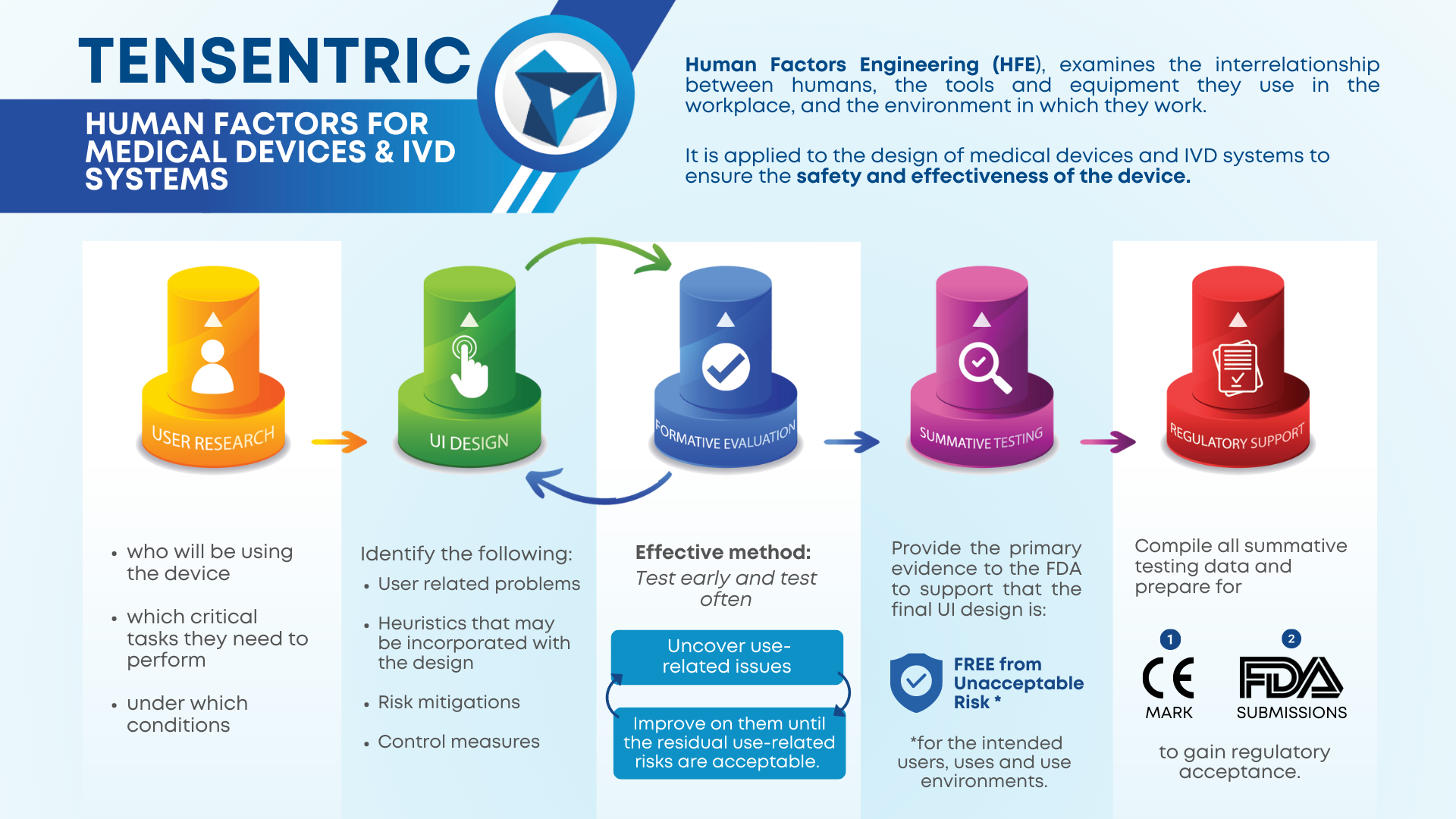
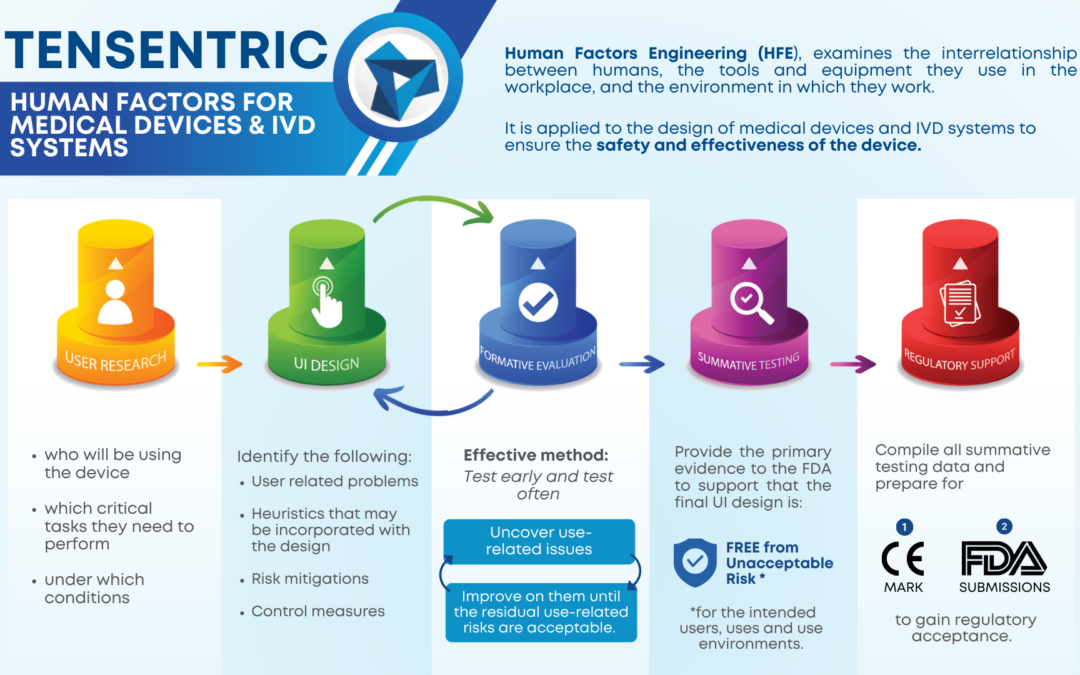
USER RESEARCH
The HF process begins with user research to gain an understanding of the context of use: who will be using the device, which critical tasks they need to perform, and under which conditions. This will help to identify environmental, social, and motivational considerations for the device design so that the user needs are met and use errors are minimized. User research activities focus on identifying relevant UI design standards, similar and predicate devices, user populations, their characteristics, their limitations, use environments, workflow and critical tasks, and may be conducted through point-of-use studies, surveys, interviews, and/or field observations.
UI DESIGN
Once the user research is complete, anticipated use-related problems and relevant heuristics may be incorporated into the concept designs, along with any risk mitigations and control measures. It is important to iterate through formative evaluations during the UI design process, applying any feedback or results from the formative evaluations into the UI design.
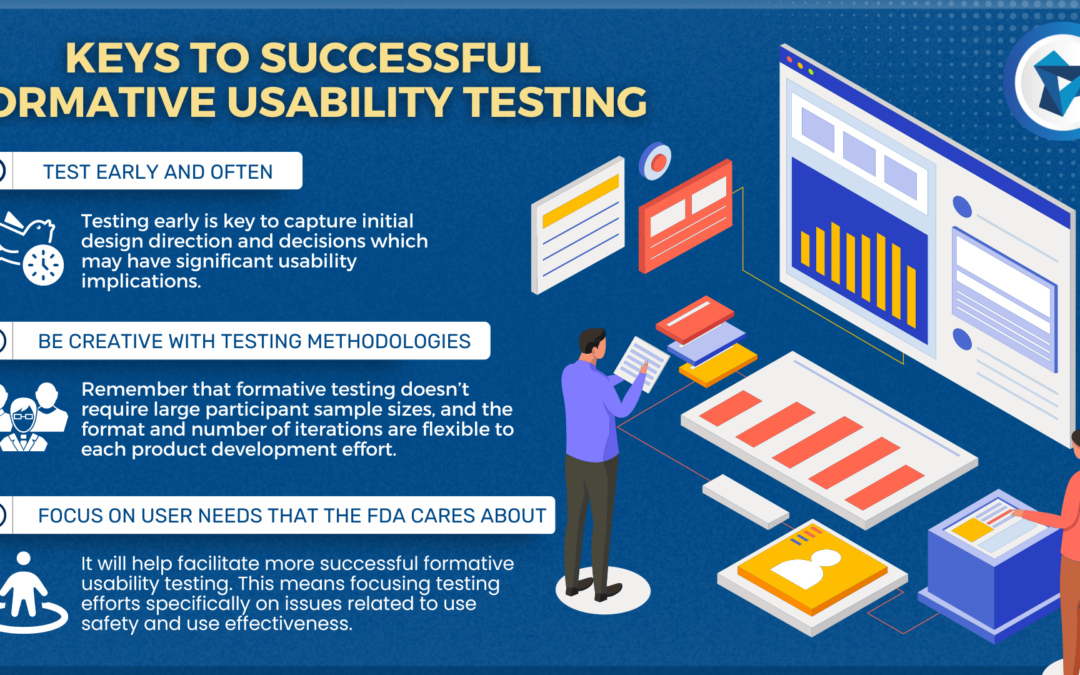
FORMATIVE EVALUATIONS
The most important aspect of formative evaluations is to test early and test often. Formative evaluations are a detection method used to uncover use-related issues and iteratively improve on them until the residual use-related risks are acceptable. Use-related patterns can emerge within just three participants, allowing the nature of formative evaluations to be less formal, with in-house testing being a viable option for early tests, although using the intended users as participants is often most effective. Overall, the goal of formative evaluations is to validate the safety and effectiveness of the device so that the summative test plan can be finalized and ready to execute.
SUMMATIVE TESTING
The outcomes of summative testing provide the primary evidence to the FDA to support that the final UI design is free from unacceptable risk for the intended users, uses and use environments. After completing summative testing, a report should be complied that documents the purpose of the test, the testing conditions, objective (performance) and subjective (participant’s assessment) data, observed use errors with applicable explanations for the errors, and a mitigation strategy for observed use errors, if any.
REGULATORY SUPPORT
The final step of the HF process is preparing for CE-Mark or FDA submissions by compiling all summative testing data and finalizing the submission documents in order to fain regulatory acceptance. If the regulatory bodies respond with AI Letters, be prepared to give a timely and truthful response, otherwise begin preparing post-market surveillance strategies with your newly approved device!
WHAT IS THE ROI?
You may be thinking, “This sounds nice and all… but is it worth the added time and money? What are the benefits?” First of all, the FDA now requires that HF be thoroughly applied to all medical device design and development. But you are in luck – applying HF early to your design actually gives a positive ROI because of the following reasons:
- Increased sales from greater ease of use, enabling better competition in the marketplace
- Faster time to market with less late-stage design changes due to missed or overlooked user needs and unmitigated use errors
- Decreased support costs when products are easier to learn, use and troubleshoot
- Simplified learning tools such as training and IFUs when products are designed for the intended users’ capabilities
- New design concepts identified in user research and iterative formative evaluations that meet the user’s needs
- Longer design life when products are optimized for their intended user and remain competitive for longer periods of time
SUMMARY
- Start the HF process early in the design process, beginning with user research.
- Let the user-centered data drive your device design.
- Applying the HF process to your device design early is always worth the investment and will ensure you create safe and effective devices.