When designing a new medical, diagnostic, or life science device, the safe and effective delivery of product is just as important as its safe and effective use. Often there are several levels of packaging, one for it to be shipped in bulk, like a crate, one for it to be shipped in small quantities, like a box, and another for the end user, like a plastic bag. Each layer of packaging serves a specific purpose in reliable delivery and use of the product.
Packaging design is an integral part of a new or existing product. Tensentric will help you realize the complete vision of your product, in-and-out-side the box.
ESTABLISHING REQUIREMENTS FOR YOUR PRODUCT
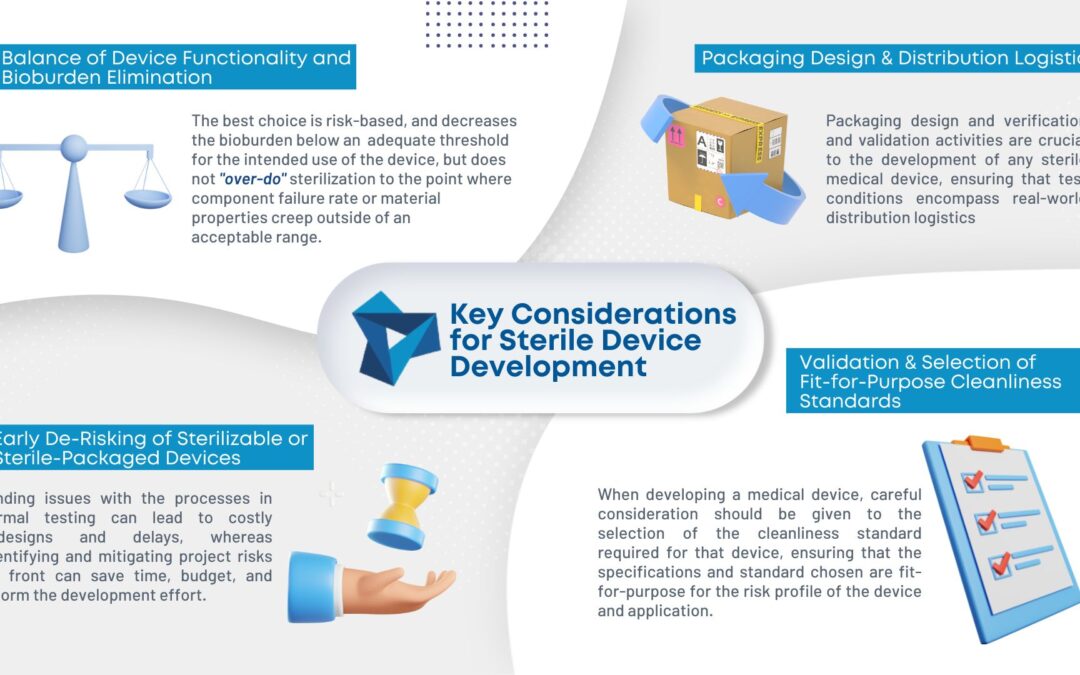
Among the key considerations for packaging design are product integrity, package integrity, cost, manufacturability, and usability. Beyond the regular strains of shipping (shock, vibration, and climactic stress), medical packaging may be uniquely exposed to sterilization (gamma radiation, E-beam, ETO, steam), extreme storage conditions (incubator, freezer, storage shelf), unpacking constraints (gloved hands, particulate sensitivity, cleaning procedures), and final usage requirements (functional features, identifying labels, use instructions). Materials, manufacturing methods, and packaging construction must be compatible with the processes that the product will be exposed to from manufacture through use. Developing a set of packaging requirements that can be tested and verified alongside the product requirements ensures a well-integrated system that works as intended after distribution.
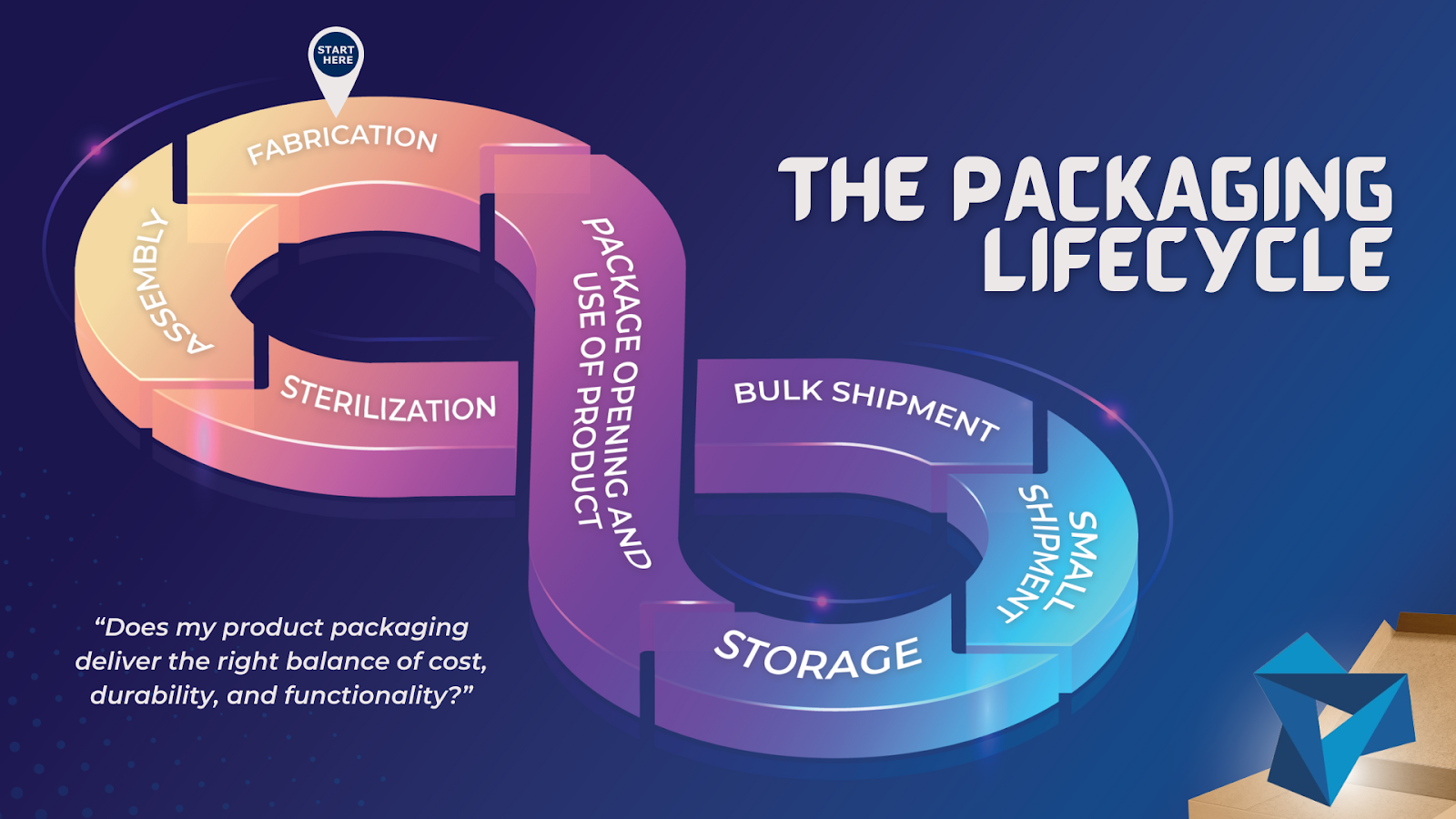
THE PACKAGING LIFECYCLE
Ask yourself, “does my product packaging deliver the right balance of cost, durability, and functionality?” in:
- Fabrication
- Assembly
- Sterilization (if applicable)
- Bulk shipment (from manufacturer to client)
- Small shipment (from client to customer)
- Storage (at the laboratory, hospital, office, or home)
- Package opening and use of product
IDENTIFYING IF YOU NEED NEW PACKAGING
Packaging is frequently only revisited when the product is updated, but if the packaging is causing issues, it’s best to redesign it before more product is damaged. There are several issues to look out for.
- Product damage, including rubbing, scratches, etc.
- Sterile barrier damage
- Excessive assembly time or component costs
- Users struggle to open or damage the product while opening
- Users have trouble locating pieces or directions
ROBUST PACKAGING REDESIGNS
Your current packaging might seem like the inexpensive or easy choice, but this isn’t always the case, especially if the product or its environment has changed. If a product is being damaged during shipping it can quickly cost more in replacement parts and time than a dedicated packaging redesign. Tensentric uses an iterative design process to prototype and test packaging solutions, exposing issues early in the design cycle and correcting them before moving into production. If sterilization and cleaning are a factor, we will use appropriate materials for the process then assemble in our ISO Class 8 clean room.
USABILITY PACKAGING REDESIGNS
If a product comes in a kit with multiple components and/or instructions, it can be a complex usability test. Intentional packaging can augment and guide the experience. Progressive disclosure, where the user only sees the components relevant to the active step, simplifies a complex process into discrete steps and creates a more intuitive association of components with those steps. Labeling, color coding, and assistive or indicative features within the packaging also encourage safe and effective use of the product.
KEY POINTS
- Approach product packaging design as you would the product itself
- Understand when it’s time for new packaging (if developing a new product, the time is now!)
- Determine your product’s packaging needs, then design and test against them
- Understand the user’s needs that may influence the packaging design