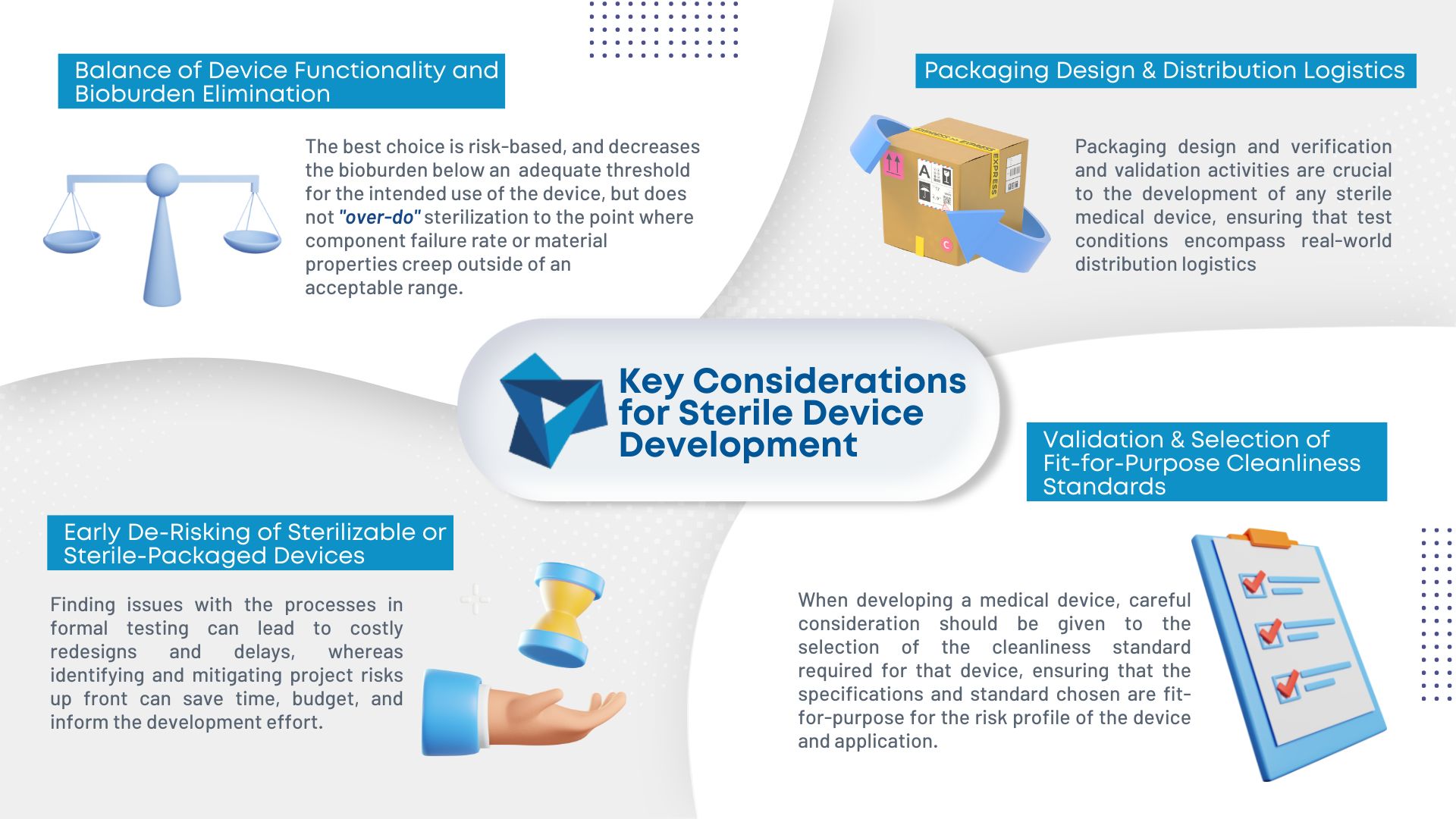
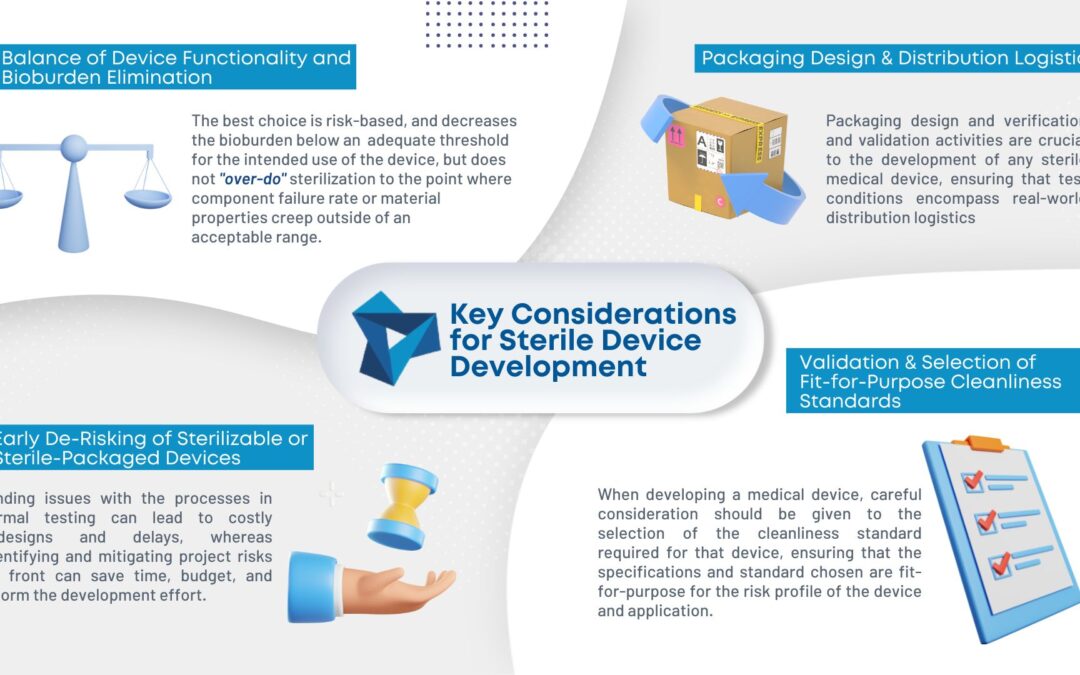
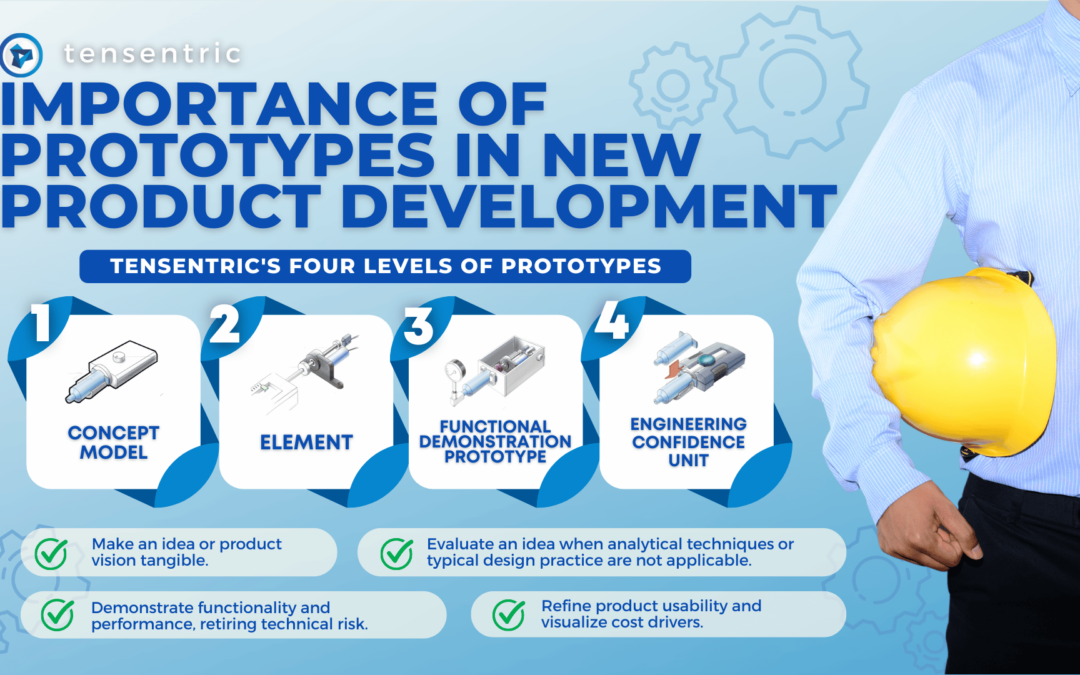
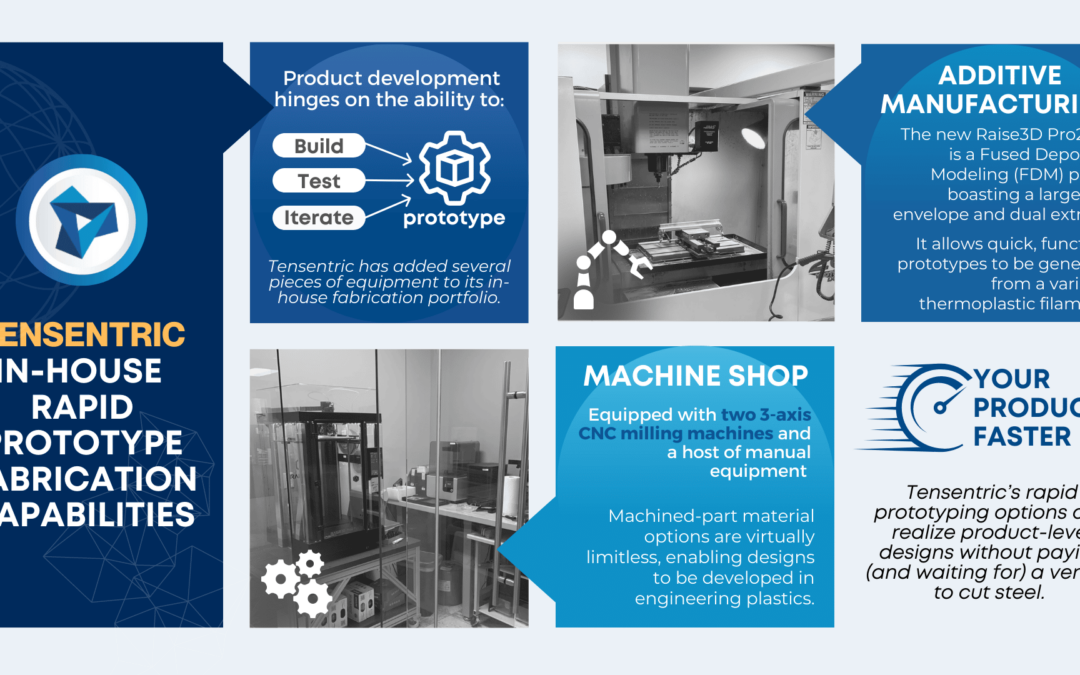
As medical device consumables become more complex, so does manufacturing them. Sterilization is becoming an increasing concern in the industry. To ensure the proper sterilization is in place, there are four key considerations during development:
- Balance of device functionality and bioburden elimination: Different sterilization methods have different degradation effects on materials making it important to evaluate the risks and decrease bioburden.
- Packaging design and distribution logistics: A sterile-packaged device is only as good as the packaging that maintains its sterility.
- Early de-risking of sterilizable or sterile-packaged devices: Performing informal testing during prototyping can ensure the design meets all requirements and minimize formal testing down the line.
- Validation and selection of fit-for-purpose cleanliness standards: The required cleanliness standard for the device and application should be heavily considered during the development stage.
Manufacturing saleable medical devices and in-vitro diagnostics is Tensentric’s core competency. Learn more about working with our team.