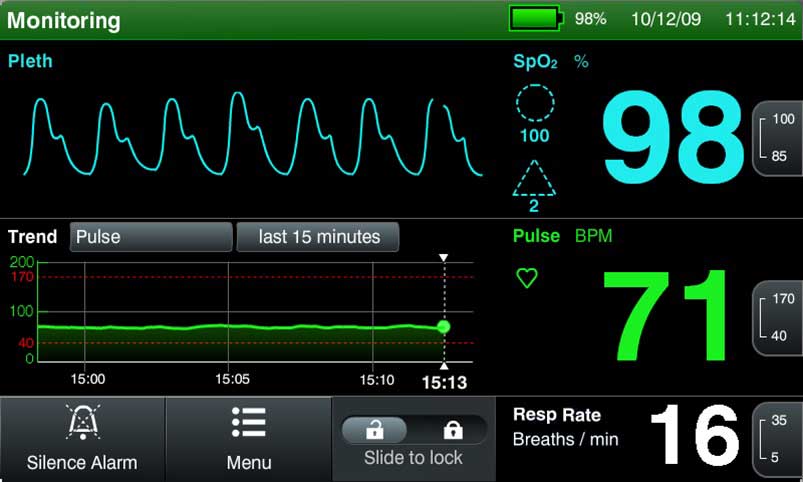
Designing in-vitro diagnostic (IVD) systems is an inherently cross-disciplinary effort, as most systems exist at the convergence of technology, biology, and chemistry. Although IVD systems do not operate in direct contact with patients, these instruments and disposables also require the kind of attention to biology we afford to other medical devices. The results of diagnostic tests allow patients to have insights into their medical condition, informing treatment and lifestyle decisions. For the development of a precise and accurate diagnostic system, the interfaces between biochemistry and mechanical, electrical, and optical subsystems must be fully defined. To accomplish this, one thing is imperative: don’t forget the assay!

Although IVD Systems do not operate in direct contact with patients, these instruments and disposables also require the kind of attention to biology we afford to other medical devices.
IVD SYSTEM DESIGN STARTS WITH THE CHEMISTRY
Diagnostic devices operate by producing an output of information based on the technological analysis or characterization of an input biological sample. In IVD systems which utilize chemical assays, a specific chemistry produces a change in the biological sample that can be detected by the device. Therefore, it is critical for any IVD device design to start with understanding the chemical assay and the biological sample with which the device will need to interface.
This understanding starts at the lab bench. Before designing a cartridge or other disposable, the assay procedure needs to be understood, including any reagents, volumes, temperatures, shaking, etc. Assay requirements feed directly into device design decisions. For example, if the assay is sensitive to temperature fluctuations, active temperature control may be required. If the sample integrity could be at risk in environments with high shear stress, certain modes of driving the fluidics of the system may be eliminated. Certain reagents may impose material compatibility requirements. Even the timing of the assay is important to understand so that the intended use of the devices and the user workflow can be accurately defined. Does the user input the patient sample and push ‘go’, or are more interventions required? Is the diagnostic likely to be a point-of-care device or does the chemistry take longer than a typical patient visit? These types of questions are all critical to understand from the bench before attempting to move the chemistry into a device.
Ultimately, knowing what the disposable and instrument need to bring to the table in order for the assay to function as designed is an essential step in going from bench top science to a clinical product. The only way to discern the requirements of the IVD system is to understand the functional needs and limitations of the biological sample and the chemical assay.
FREQUENT ASSAY TESTING DURING PROTOTPYING DE-RISKS PRODUCT DEVELOPMENT
Having in-house capabilities to run actual samples (such as a BSL-2 laboratory) can significantly de-risk product development. Major device architecture decisions are often based on assay needs; testing of these architecture elements and directions with biological samples using the actual assay chemistry is vital to inform choices early in product development. Don’t assume just because the chemistry worked in a well-plate in a curated laboratory environment that it will work on any device design as long as the right chemicals are present at the right time.
Even if a chosen product architecture made sense at the beginning of a product development effort and was demonstrated to work with the assay, subsequent untested small changes to the design could have profound implications on assay performance and accuracy. Testing designs and prototypes frequently with real biological samples means that design changes that have adverse effects on diagnostic performance are more easily identified and fixed before confounding changes cause major delays while decreased assay performance is investigated. This allows a more complete understanding of the theoretical factors which impact the assay as well, leading to better future design decisions.
Frequent testing of prototypes with actual biological samples not only de-risks product development as described above, but also accelerates design iterations by closing the feedback loop between engineers and scientists. When engineers have an idea of a solution, in-house laboratory capabilities during device development allow that solution to be tested immediately so that the design can be continuously improved based on a true measure of performance.
SUMMARY
- The biochemical function of a specific diagnostic assay drives the instrument and disposable requirements.
- The electrical, mechanical, and optical components of in-vitro diagnostic systems should be developed in tandem with the chemistry and biology to ensure that the assay will successfully move from bench to bedside.
- In-house laboratory capabilities allow medical device designers to de-risk design concepts earlier and faster, accelerating product development and providing more robust systems.
Tensentric has a biosafety level 2 (BSL-2) cell-culture capable lab to support our clients’ needs for rapid testing of prototypes and design concepts for systems which interface with biological samples, tightening the design feedback loop without the need for clients to spend time and money supporting engineering efforts with testing.