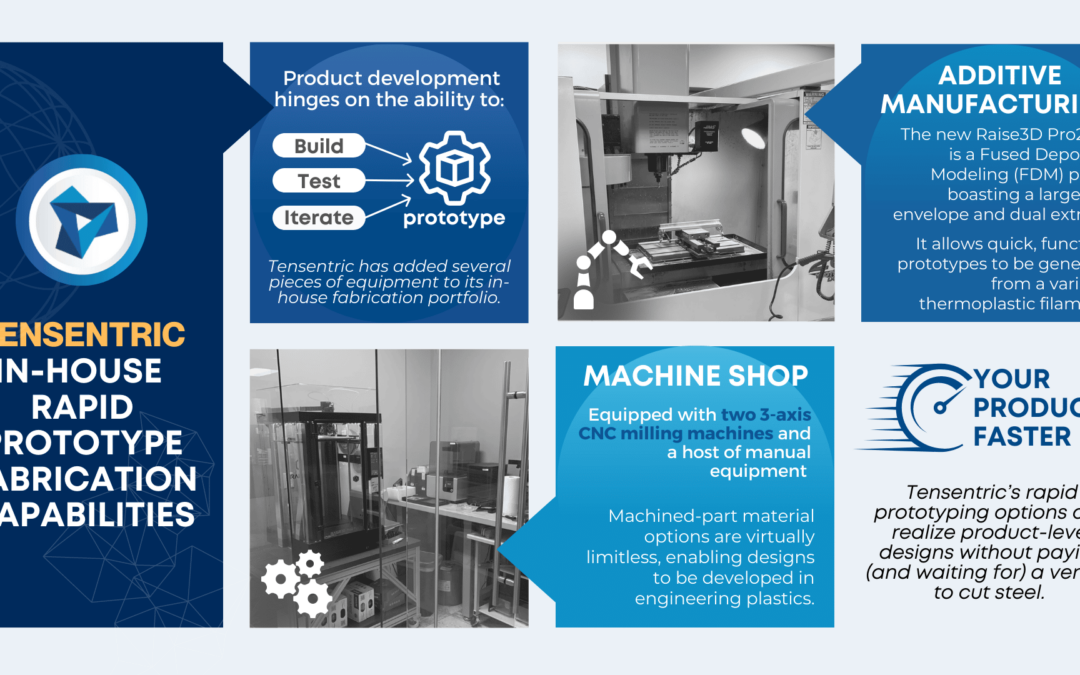
Connecting medical devices to the cloud provides tremendous advantages, not only for analysis and diagnosis, but also for maintenance and service. For home use or other point-of-care applications, using Cellular IoT technology may be the only way to make the connection.
This paper discusses some of the things you need to consider when designing a medical device with Cellular IoT connectivity.
It is important to select a cellular module that has already been certified for the countries where the product will be sold and is also certified by the wireless carrier (carriers) being considered.
HARDWARE CONSIDERATIONS
The first question to ask is where the device will be sold. Selling in US only, North America, North America and Europe, or Global markets all have different implications for the selection of which cellular technology and approach may be the best choice.
Low power wide area (LPWA) cellular IoT is the most cost-effective technology for sending small quantities of data over a cellular network. Low power wide area (LPWA) cellular IoT is split into two competing technologies: LTE Cat-M1 (LTE-M) and Narrowband Internet of Things (NB-IoT). Although North America carriers have adopted both LTE-M and NB-IoT, several European cellular carriers such as T-Mobile and Vodaphone only support NB-IoT.
Other constraints that should be considered include size and power. Cellular chips and modules come in a wide variety of sizes and many require significant power when in use. A lot of attention needs to be paid to power consumption if the device is battery powered.
Tensentric has recently designed a handheld Remote Patient Monitoring device for one of our clients that is intended for both outpatient home use by the patient and within a hospital diagnostic setting. The device design is extremely size constrained, and we selected the Nordic Semiconductor nRF9160, the smallest and lowest power Cat-M1/NB-IoT cellular module available. Tensentric has guided this design through FCC, EMC and Safety and Verizon Certifications.
CERTIFICATION
Pre-Certified cellular modules are tested and approved by the FCC (or EU) and wireless carriers to conform to low-level RF requirements. It is important to select a cellular module that has already been certified for the countries where the product will be sold and is also certified by the wireless carrier (carriers) being considered.
Getting certified for a cellular carrier is a time-consuming process and can be expensive. Currently, in the United States, getting certified by AT&T requires getting a PTCRB certification which typically cost between $25,000 to $38,000. In contrast, Verizon provides its own device certification, and this has been free for the last several years. Getting started with Verizon is a less expensive path for initial product certification and deployment.
Global Certification Forum (GCF) certification is usually required for products launched in Europe, Asia and other parts of the world. Test cases for GCF and PTCRB certification are very similar since they both test conformance to 3GPP standards.
SOFTWARE CONSIDERATIONS
Privacy is a significant concern whenever sending patient information over the internet. In addition to all the storage and access controls at each end of the connection, the data must be kept secure in transit. This means encryption, and the best two options out there are to set up a VPN, or to use HTTPS.
Data usage is of particular interest when frequently sending small amounts of data. The overhead of opening an HTTPS connection could easily dwarf the size of the actual data being transferred. In a scenario where small amounts of data need to be sent periodically, opening and closing the connection each time could create massive amounts of data overhead (and expense). Configuring the server to support open but idle connections can significantly reduce the overhead (and related data expense).
KEY POINTS
- Understand Target Markets and Certification Requirements (Market & Carrier)
- Consider Size and Power Constraints when Selecting Cellular Module
- Don’t Underestimate Certification Costs and Especially Time Required
- Software Architecture Should Consider Data Size, Frequency, Cost and Security Requirements