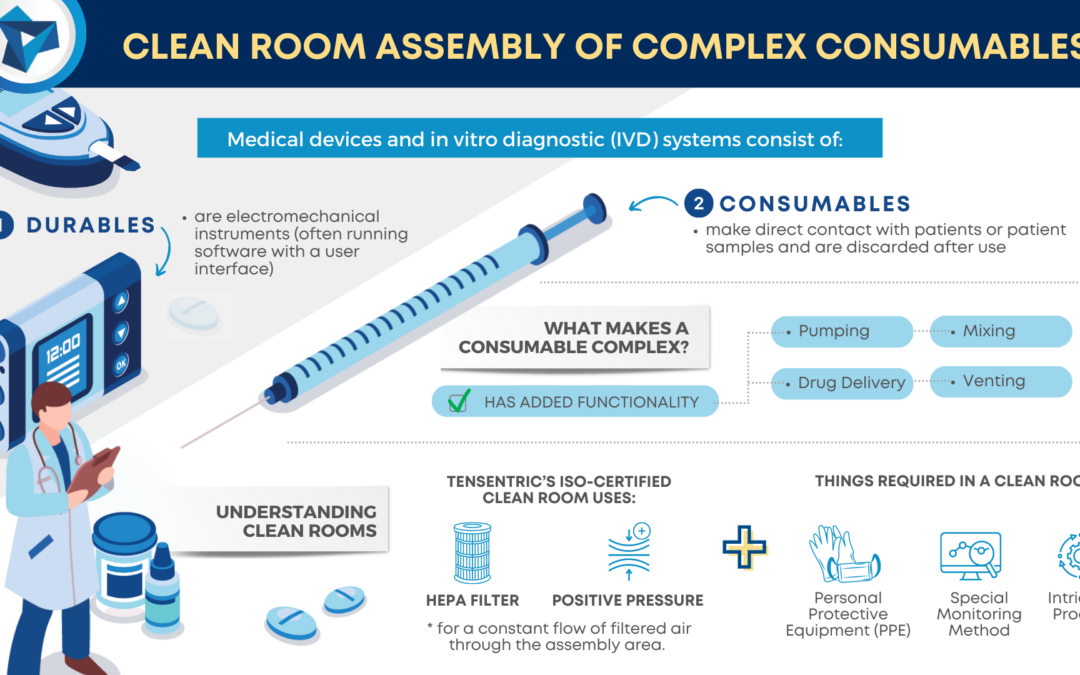
Medical devices and in vitro diagnostic (IVD) systems often consist of both durable and consumable components where the durables are reusable electromechanical instruments (often running software with a user interface) while the clean or sterile consumables make direct contact with patients or patient samples and are discarded after use.
For complex consumables requiring assembly in a clean room, Tensentric’s experienced manufacturing team is here to help bring your product to market.
WHAT MAKES A CONSUMABLE COMPLEX?
Consumables are considered complex when they consist of more than a handful of components and the manufacturing process requires a significant number of assembly steps. These kinds of consumables have added functionality such as pumping, mixing, venting, reagent or drug delivery, and more. Component tolerances tend to be tight, requiring precision fabrication methods and a stringent inspection process.
These kinds of consumables have added functionality such as pumping, mixing, venting, reagent or drug delivery, and more.
ASSEMBLY & TEST PROCESS
Any challenging assembly or alignment steps may require custom fixtures to be designed, built, and verified to assist the assembler by holding the product in place. Assembly tools such as electric torque drivers are calibrated or verified before use and may require a narrow range of operation to ensure the consumables are properly fastened without causing excessive mechanical stress. After assembly, consumables go through final functional test (where possible) to confirm proper functionality before packaging and labeling.
WORKING WITH REAGENTS
IVD systems are trending toward more point of care (POC) and remote patient diagnostics that require automated sample preparation. Consumables for these types of systems often have on-board reagents that are used to mix with and prepare the sample for measurement or detection. Design considerations for consumables with onboard reagents include not just the fluidics, mixing, and functionality but also significant manufacturability issues such as filling systems (accuracy and reliability), sealing (foil type and application), shelf life (material compatibility and permeability), packaging and shipping (regulatory requirements), and of course automation.
UNDERSTANDING CLEAN ROOMS

If the consumables require assembly in a clean room, there are additional steps required to prevent contamination. Tensentric’s ISO certified clean room uses HEPA filters and positive pressure for a constant flow of filtered air through the assembly area. The room is recertified annually and regularly monitored for particulate levels. Trained personnel follow cleaning processes and apply PPE in the gowning area before entering the room. Equipment and materials also go through a cleaning process before they can be transferred into the clean room. Product components are either fabricated in a clean environment or go through a validated cleaning process.
Once the consumables are assembled, tested, and packaged, they can be safely transferred out of the clean room and either sent to a sterilization facility for processing or shipped through distribution channels for use. When build activities complete for the day, personnel conduct a multilevel cleaning process to ensure the room is ready for the next build.
SUMMARY
- Complex consumables have added functionality and require a higher number of assembly steps, often with precision components
- Custom fixtures can assist manufacturing personnel during challenging assembly steps
- On-board reagents require filling, sealing, packaging, and shelf-life considerations
- Clean rooms require special equipment, PPE, processes, and monitoring methods to prevent contamination of product
Tensentric’s FDA registered and ISO 13485:2016 facility in Louisville, CO is manufacturing complex consumables in its ISO certified clean room for the medical, IVD, and life sciences industries.