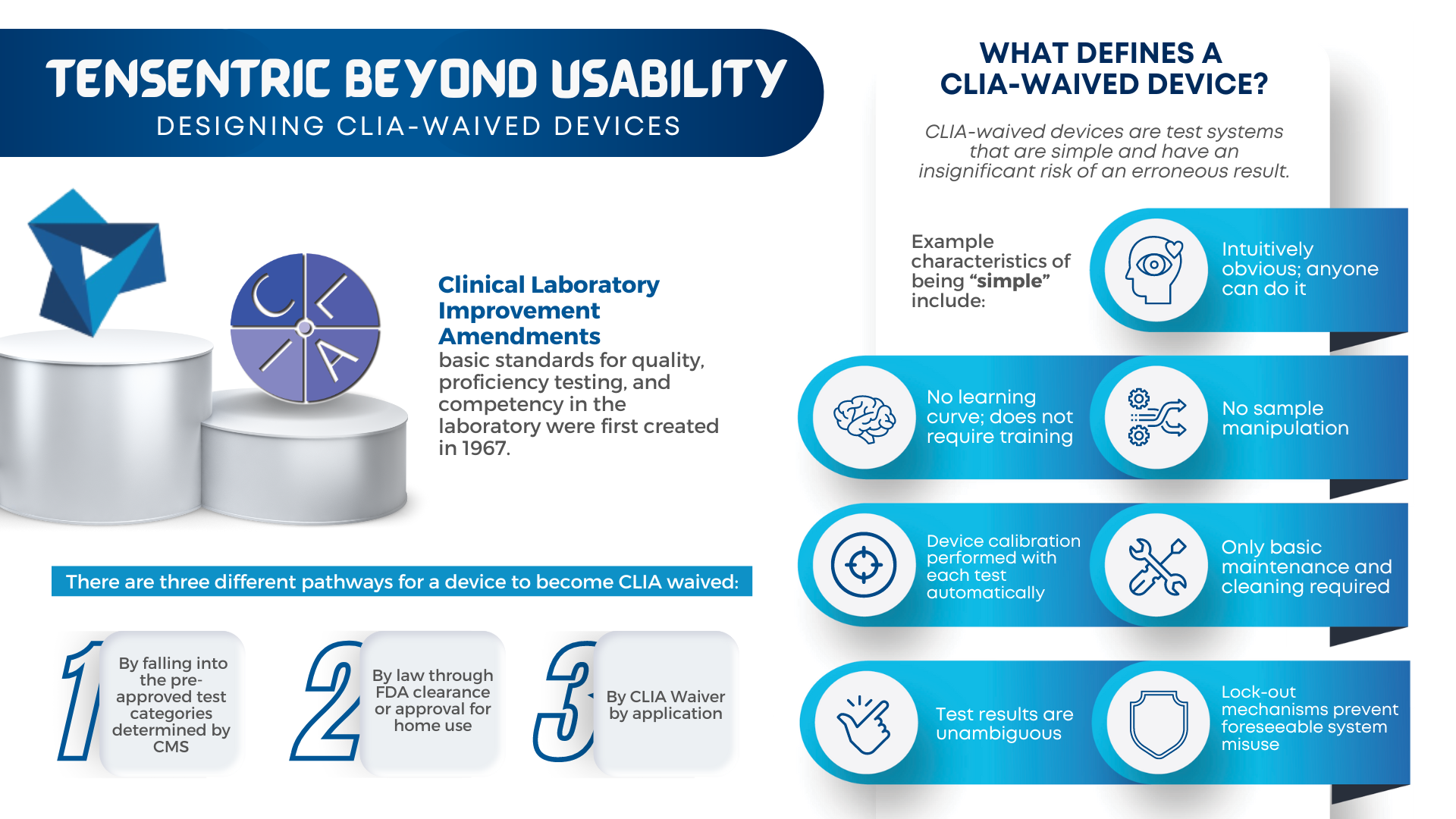
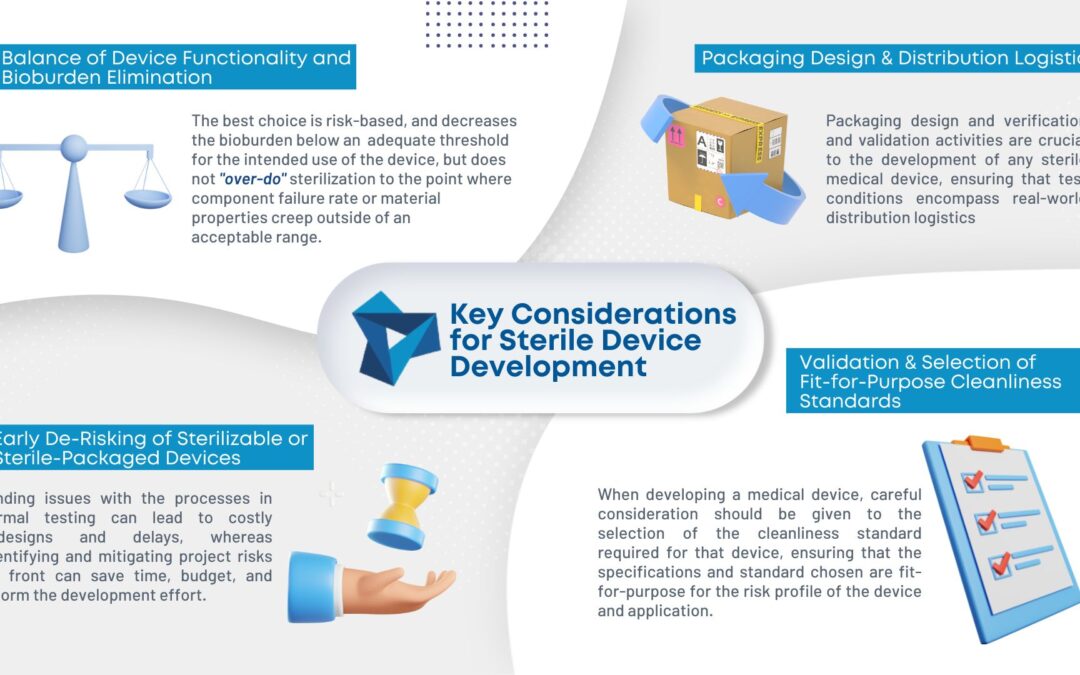
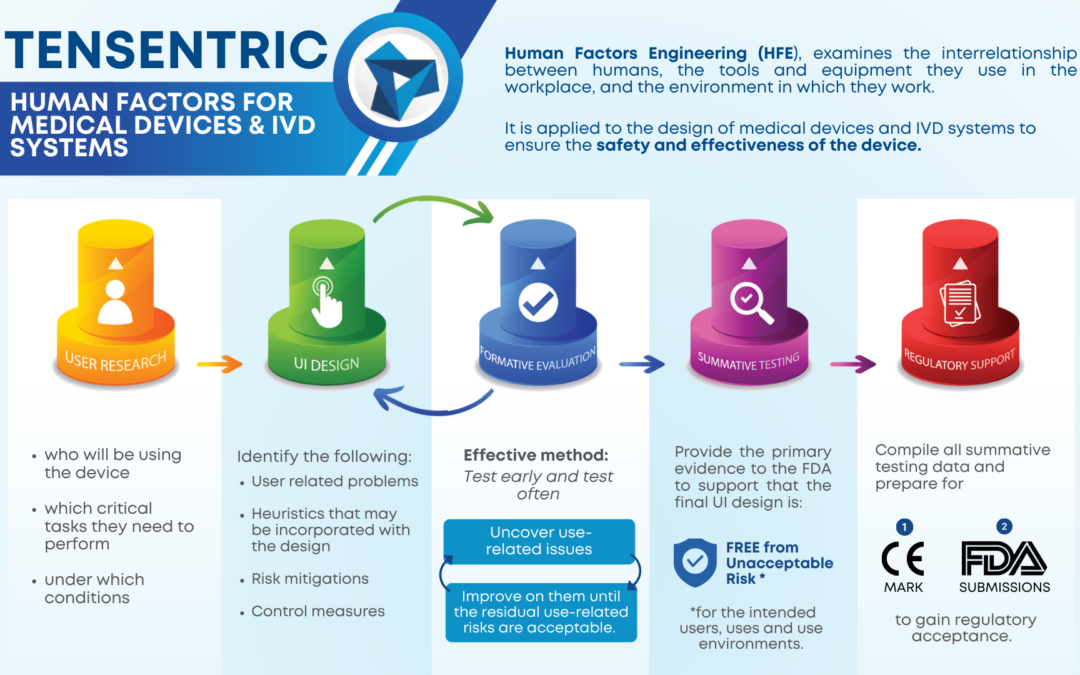
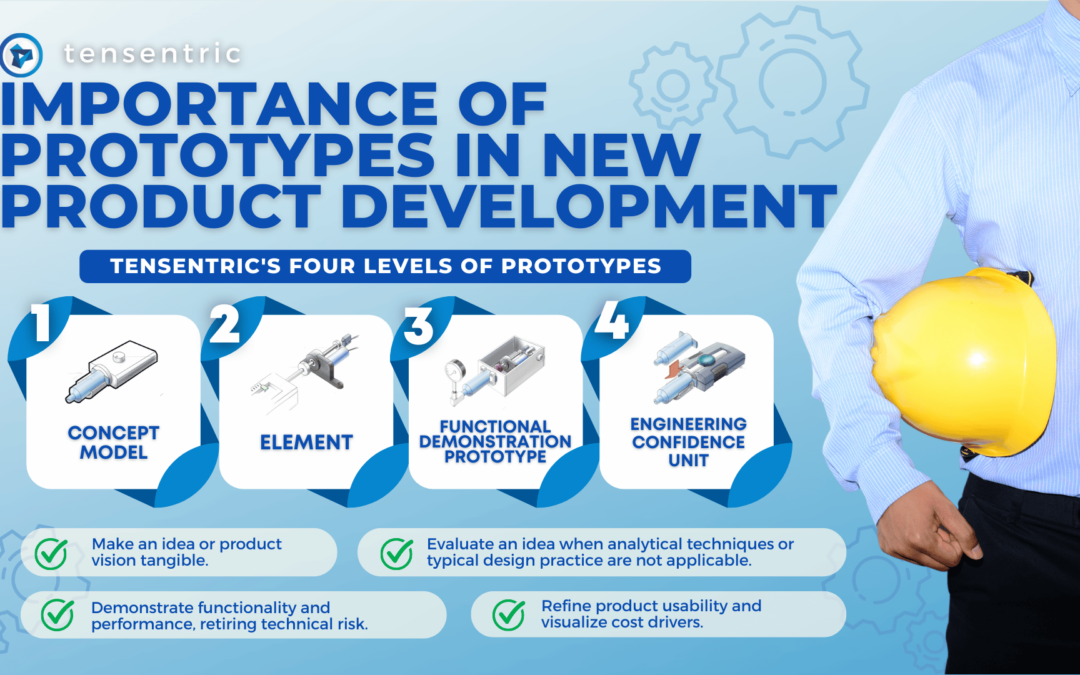
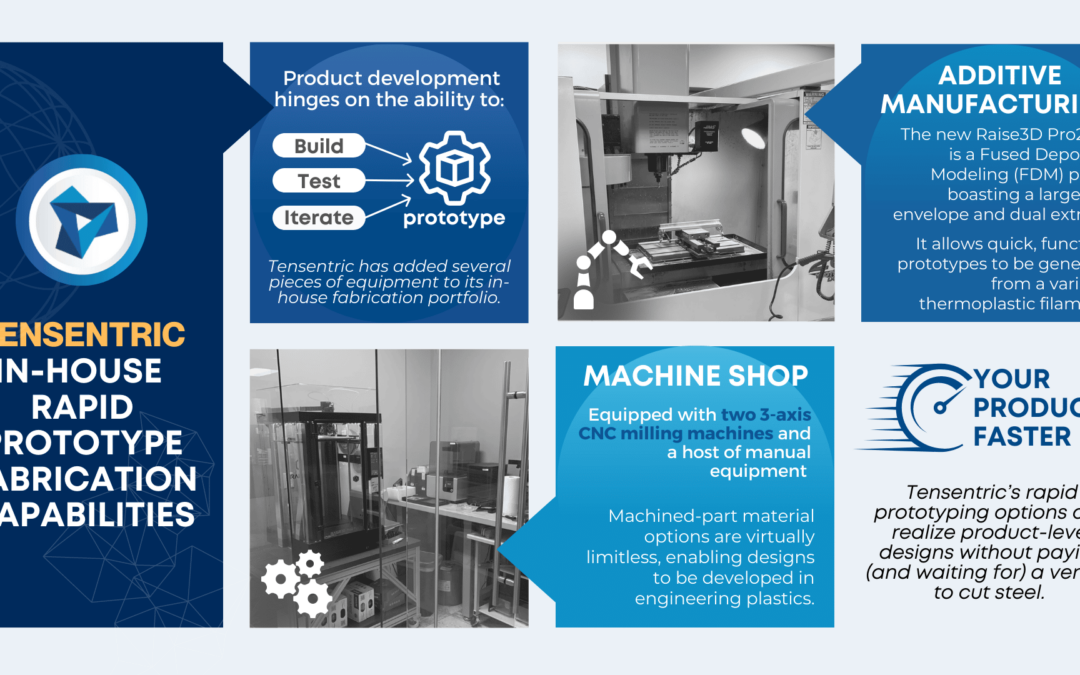
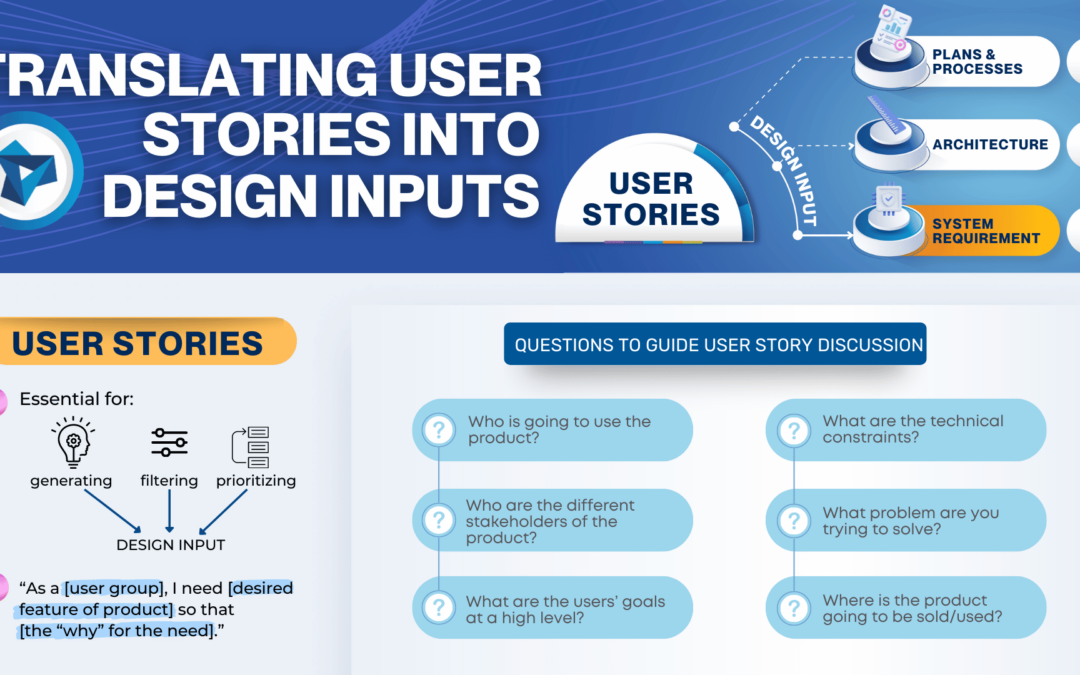
Are you ready for the Clinical Laboratory Improvement Amendments waiver? With the large market for laboratory devices growing fast, the benefits of designing for the waiver are even more important for better and faster patient care.
For manufacturers, it vastly broadens the potential market to include non-lab and non-hospital clinics, urgent care centers, medical facilities with a Certificate of Waiver and even home care. For patients and providers, it improves patient care by streamlining urgent testing with access to near-immediate test results for in-office diagnosis and treatment.
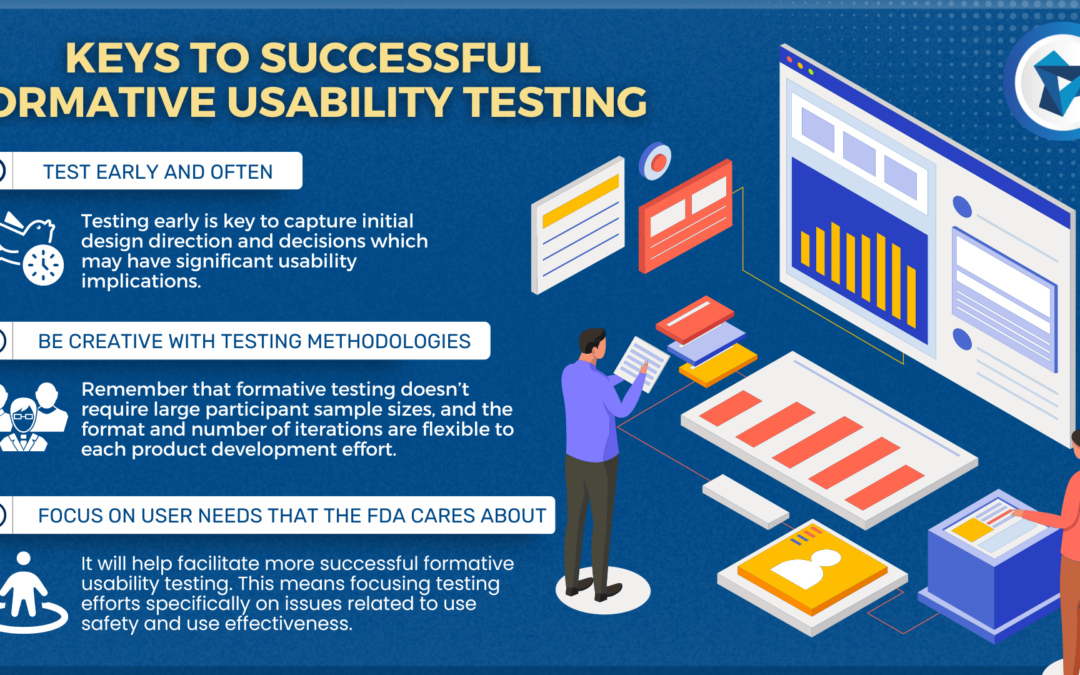
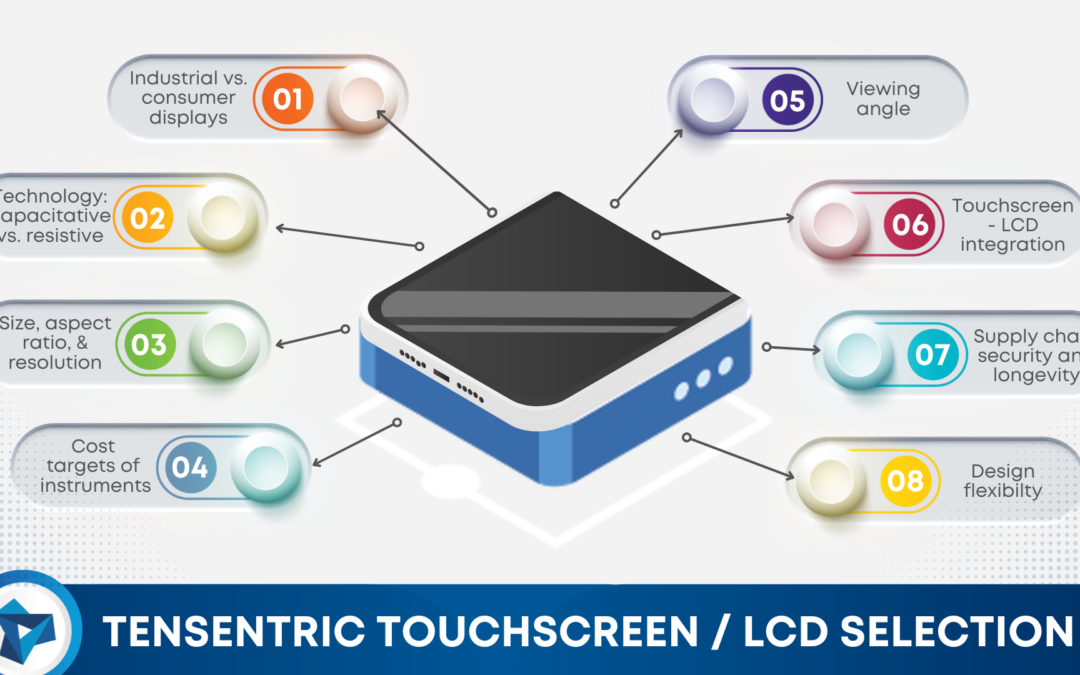
Learn more about how our team is equipped to support CLIA-waived device design efforts with years of experience designing simple and accurate devices that meet the waived-test criteria set forth by the FDA.