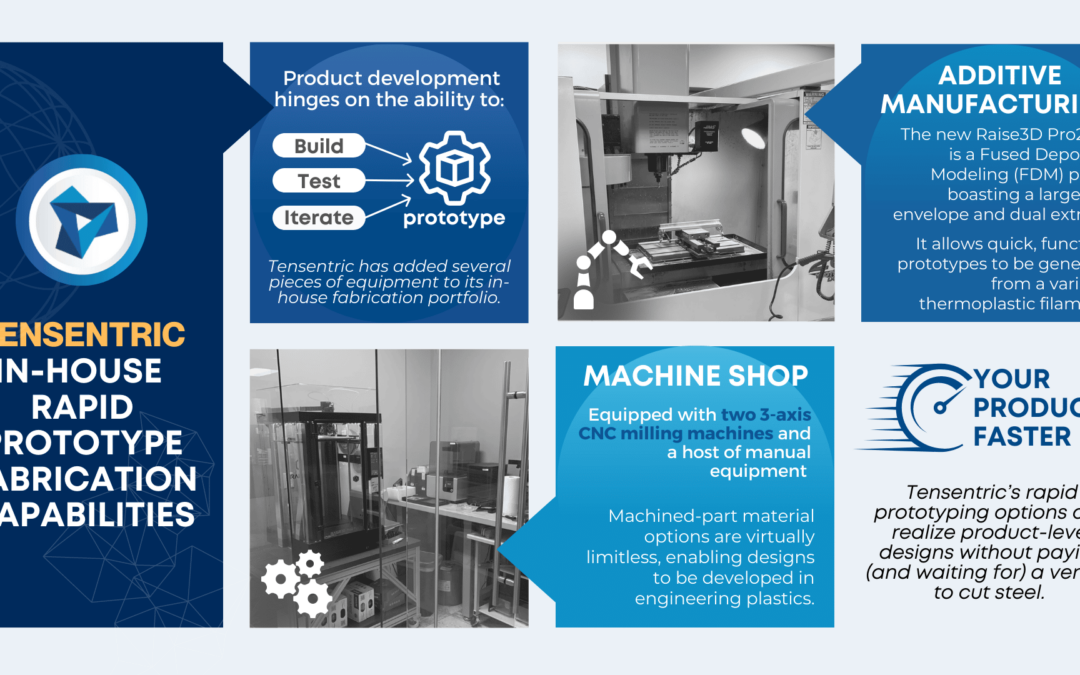
Product development hinges on the ability to build, test, and iterate upon prototypes. To increase the speed at which we go from digital to physical, Tensentric has added several pieces of equipment to its in-house fabrication portfolio.
MACHINE SHOP
 Equipped with two 3-axis CNC milling machines and a host of manual equipment including a lathe and surface grinder, our machinist (38 years of experience, 29 medical) can tackle projects spanning from precision titanium implantables to large footprint aluminum heater plates. Machined part material options are virtually limitless, enabling designs to be developed in engineering plastics (Delrin, acrylic, etc.) and metals (stainless steel, aluminum, etc.) with high resolution and part quality. More importantly, these capabilities allow the design team to quickly arrive at production-equivalent components and assemblies, working closely with the machinist to establish appropriate tolerances and features early in the development cycle.
Equipped with two 3-axis CNC milling machines and a host of manual equipment including a lathe and surface grinder, our machinist (38 years of experience, 29 medical) can tackle projects spanning from precision titanium implantables to large footprint aluminum heater plates. Machined part material options are virtually limitless, enabling designs to be developed in engineering plastics (Delrin, acrylic, etc.) and metals (stainless steel, aluminum, etc.) with high resolution and part quality. More importantly, these capabilities allow the design team to quickly arrive at production-equivalent components and assemblies, working closely with the machinist to establish appropriate tolerances and features early in the development cycle.
Tensentric offers in-house machining, 3D printing, and other prototyping options to accelerate the design and development cycle with rapid iteration.
 ADDITIVE MANUFACTURING
ADDITIVE MANUFACTURING
The recent addition of the Raise3D Pro2 Plus, a Fused Deposition Modeling (FDM) printer boasting a large print envelope and dual extruder, allows quick, functional prototypes to be generated from a variety of thermoplastic filaments (ABS, PLA, TPE, etc.). Complementing the FDM printer is the formlabs Form 2, a Stereolithography (SLA) printer that produces high-resolution parts out of engineering resins. SLA prints are often the first step in assessing designs for injection molding due to their high precision and ABS/PC-like materials.
YOUR PRODUCT, FASTER
The road doesn’t end with the part – downstream applications for printed and machined models include prototyping casting patterns, rubber compression/plastic injection molds, thermoform forms, sheet metal dies, and more, allowing assemblies and manufacturing fixtures to be evaluated early. Tensentric’s rapid prototyping options can realize product-level designs without paying (and waiting for) a vendor to cut steel. Of course, when the time is right, Tensentric will leverage its supplier network and fabrication expertise to source production components, too.
Tensentric is a team of highly experienced engineers developing a wide range of medical devices and in vitro diagnostic systems. Tensentric has completed over 300 development projects for clients in the medical device and IVD space since the company’s inception in 2009 and is ISO 13485:2016 certified for design and manufacturing.